2023
158) 1-Azaspiro[3.3]heptane as a Bioisostere of Piperidine
A. A. Kirichok, H. Tkachuk, Y. Kozyriev, O. Shablykin, O. Datsenko, D. Granat, T. Yegorova, Y. P. Bas, V. Semirenko, I. Pishel, V. Kubyshkin, D. Lesyk, O. Klymenko-Ulianov, P. K. Mykhailiuk*
Angew. Chem. Int. Ed. 2023, 62, e202311583
![1-Azaspiro[3.3]heptane as a Bioisostere of Piperidine](https://mykhailiukchem.org/wp-content/uploads/2024/02/158.png)
157) 1,2-Disubstituted bicyclo[2.1.1]hexanes as saturated bioisosteres of ortho-substituted benzene
V. A. Denisenko, P. Garbuz, Y. Makovetska, O. Shablykin, D. Lesyk, G. Al-Maali, R. Korzh, I. V. Sadkova, P. K. Mykhailiuk*
Chem. Sci. 2023, 14, 14092-14099
![1,2-Disubstituted bicyclo[2.1.1]hexanes as saturated bioisosteres of ortho-substituted benzene](https://mykhailiukchem.org/wp-content/uploads/2024/02/157.png)
156) 2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
V. V. Levterov, Y. Panasiuk, K. Sahun, O. Stashkevych, V. Badlo, O. Shablykin, I. Sadkova, L. Bortnichuk, O. Klymenko-Ulianov, Y. Holota, L. Lachmann, P. Borysko, K. Horbatok, I. Bodenchuk, Y. Bas, D. Dudenko, P. K. Mykhailiuk*
Nature Commun. 2023, 14, 5608
![2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring](https://mykhailiukchem.org/wp-content/uploads/2023/10/156.png)
155) 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
A. Denisenko, P. Garbuz, N. M. Voloshchuk, Y. Holota, P. K. Mykhailiuk*
Nature Chem. 2023, 15, 1155–1163
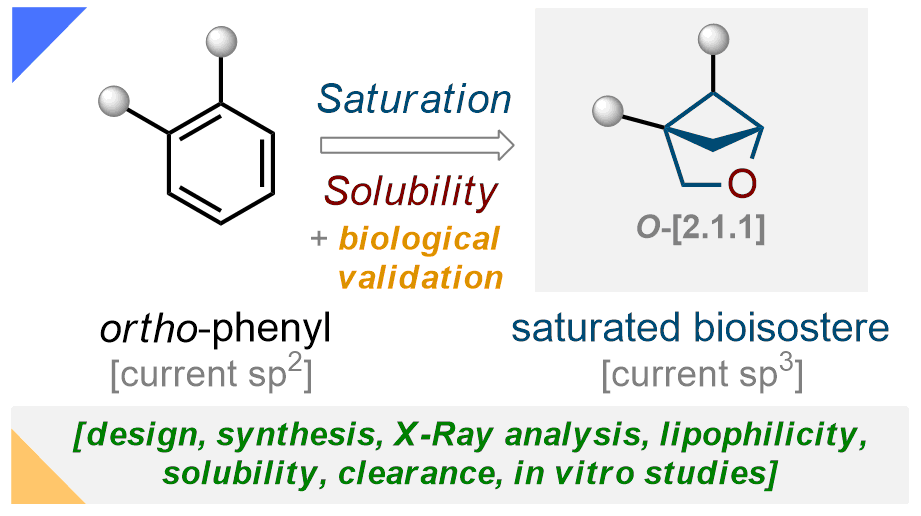
154) General Synthesis of 3-Azabicyclo[3.1.1]heptanes and Evaluation of Their Properties as Saturated Bioisosteres
D. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa, P. K. Mykhailiuk*
Angew. Chem. Int. Ed. 2023, 62, e202304246
![General Synthesis of 3-Azabicyclo[3.1.1]heptanes and Evaluation of Their Properties as Saturated Bioisosteres](https://mykhailiukchem.org/wp-content/uploads/2023/06/154.png)
153) Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes
I. F. Yu, J. L. Manske, A. Diéguez-Vázquez, A. Misale, A. E. Pashenko, P. K. Mykhailiuk, S. V. Ryabukhin, D. M. Volochnyuk, J. F. Hartwig
Nature Chem. 2023, 15, 685-693
![Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes](https://mykhailiukchem.org/wp-content/uploads/2023/06/153.png)
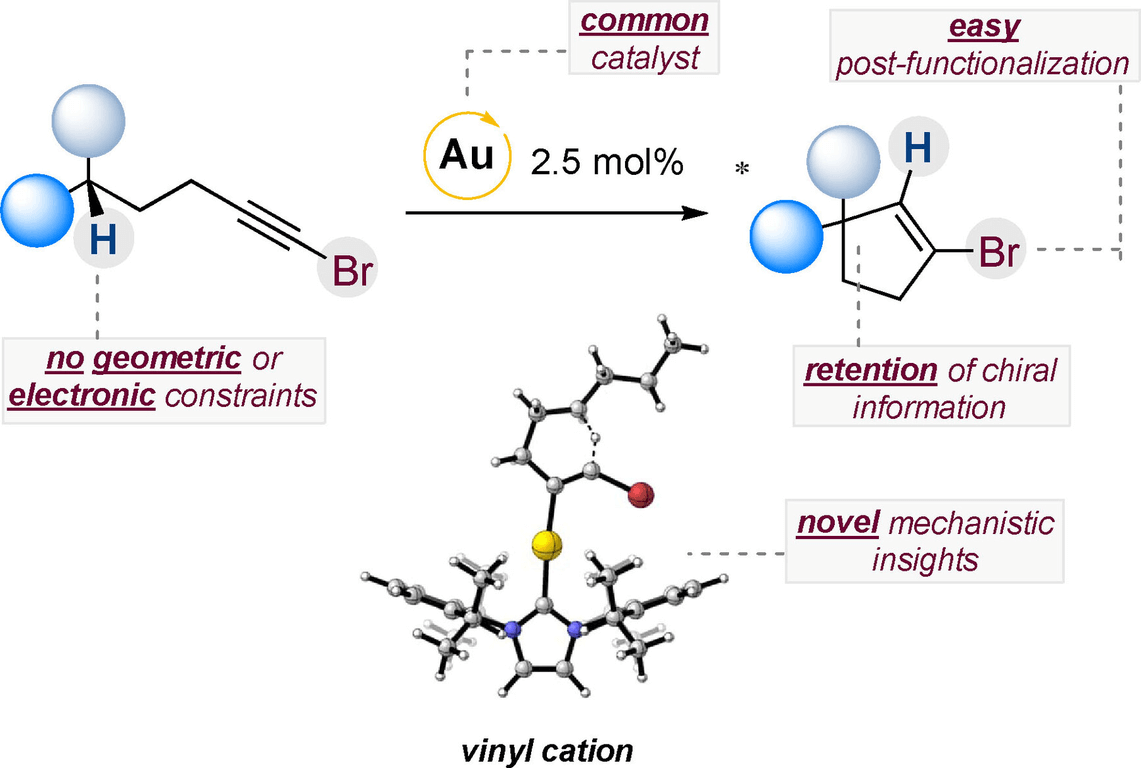
152) C−H Activation of Unbiased C(sp3)−H Bonds: Gold(I)-Catalyzed Cycloisomerization of 1-Bromoalkynes
R. Miguélez, N. Semleit, C. Rodríguez-Arias, P. Mykhailiuk, J. M. González, G. Haberhauer, P. Barrio*
Angew. Chem. Int. Ed. 2023, 62, e202305296
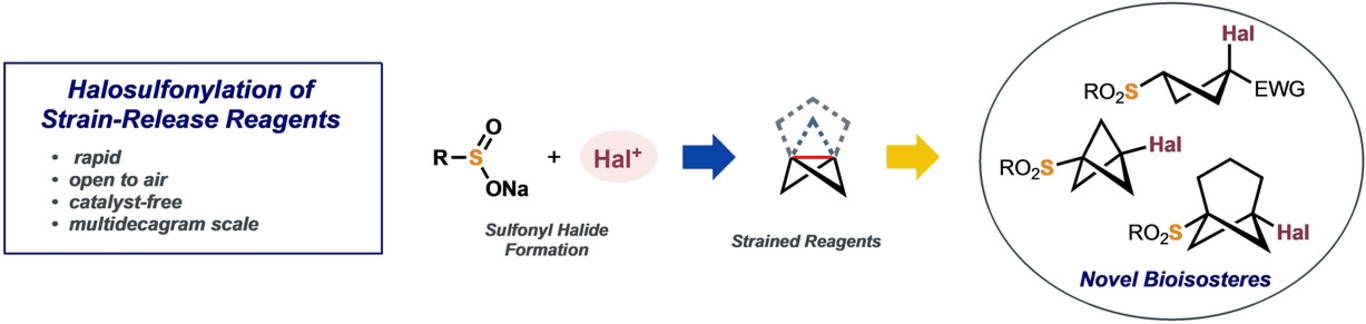
151) Rapid and scalable halosulfonylation of strain-release reagents
H. D. Pickford, V. Ripenko, R. E. McNamee, S. Holovchuk, A. L. Thompson, R. C. Smith, P. K. Mykhailiuk,* E. A. Anderson*
Angew. Chem. Int. Ed. 2023, 62, e202213508
150) General and Scalable Approach to Trifluoromethyl-Substituted Cyclopropanes
V. Ahunovych, A. A. Klipkov, M. Bugera, K. Tarasenko, S. Trofymchuk, O. Stanko, A. Boretskyi, M. Zheludenko, I. V. Sadkova, P. K. Mykhailiuk*
J. Org. Chem. 2023, 88, 3859-3870

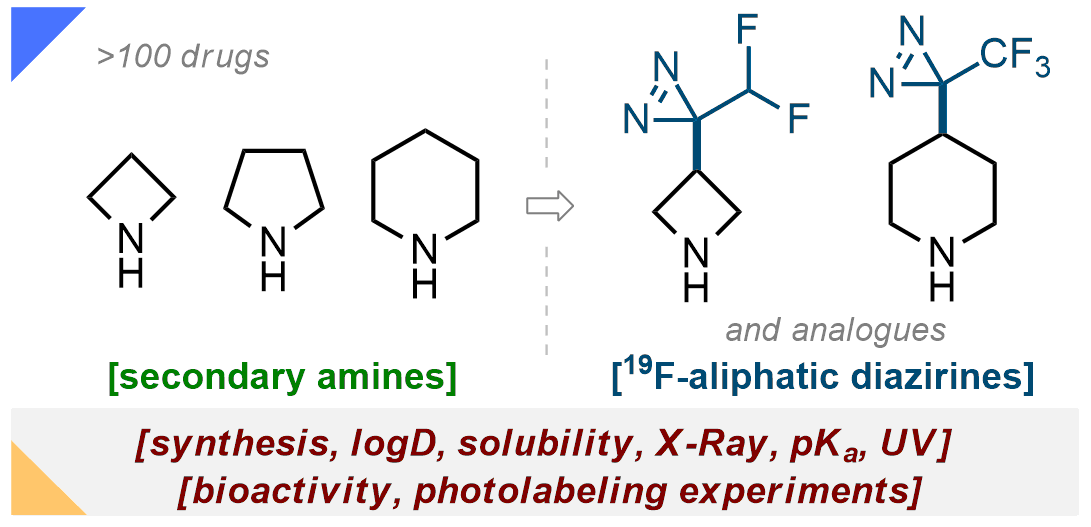
149) Fluorinated Aliphatic Diazirines: Preparation, Characterization, and Photolabeling Studies
U. Kornii, O. Shablykin, T. Tarasiuk, O. Stepaniuk, V. Matvienko, D. Aloshyn, N. Zahorodniuk, P. K. Mykhailiuk*
J. Org. Chem. 2023, 88, 1-17
2022
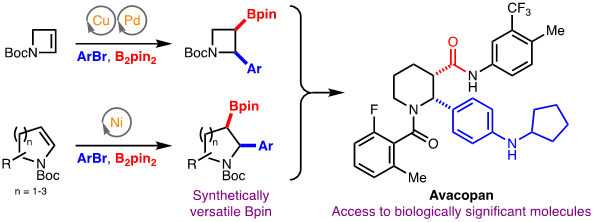
148) Arylboration of Enecarbamates for the Synthesis of Borylated Saturated N-Heterocycles
G. L. Trammel, P. B. Kannangara, D. Vasko, O. Datsenko, P. Mykhailiuk, M. K. Brown*
Angew. Chem. Int. Ed. 2022, 61, e202212117
147) A Practical and Scalable Approach to Fluoro-Substituted Bicyclo[1.1.1]pentanes
R. Bychek, P. K. Mykhailiuk*
Angew. Chem. Int. Ed. 2022, 61, e202205103
![A Practical and Scalable Approach to Fluoro-Substituted Bicyclo[1.1.1]pentanes](https://mykhailiukchem.org/wp-content/uploads/2022/10/147.png)
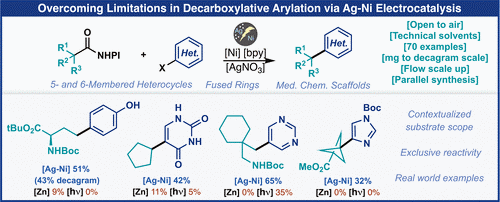
146) Overcoming Limitations in Decarboxylative Arylation via Ag–Ni Electrocatalysis
M. D. Palkowitz, G. Laudadio, S. Kolb, J. Choi, M. S. Oderinde, T. El-Hayek Ewing, P. N. Bolduc, T. Chen, H. Zhang, P. T. W. Cheng, B. Zhang, M. D. Mandler, V. D. Blasczak, J. M. Richter, M. R. Collins, R. L. Schioldager, M. Bravo, T. G. M. Dhar, B. Vokits, Y. Zhu, P.-G. Echeverria, M. A. Poss, S. A. Shaw, S. Clementson, N. N. Petersen, P. K. Mykhailiuk, P. S. Baran*
J. Am. Chem. Soc. 2022, 121, 17709-17720
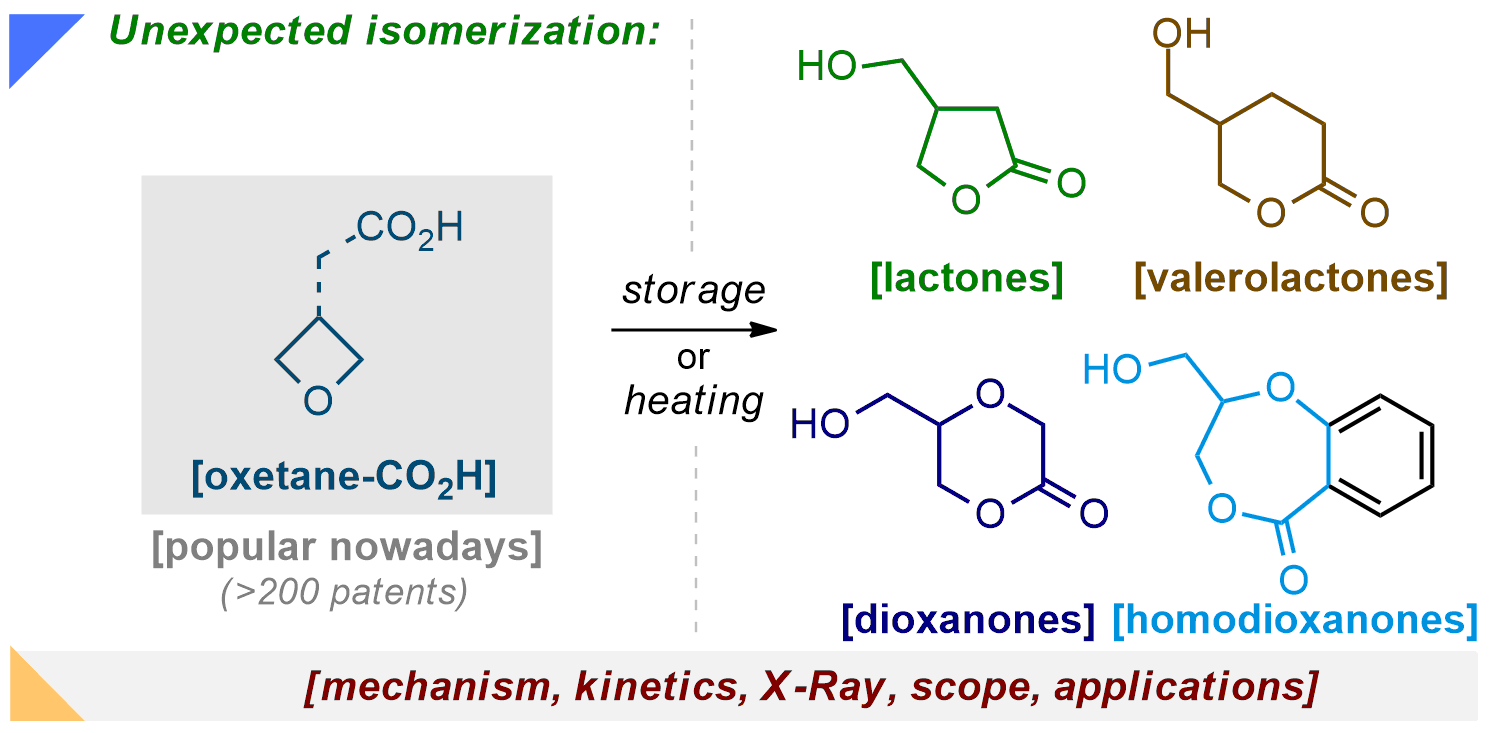
145) Unexpected Isomerization of Oxetane-Carboxylic Acids
B. Chalyk, A. Grynyova, K. Filimonova, T. V. Rudenko, D. Dibchak, P. K. Mykhailiuk*
Org. Lett. 2022, 24, 4722-4728
144) Fluorine-Containing Prolines: Synthetic Strategies, Applications, and Opportunities
P. K. Mykhailiuk*
J. Org. Chem. 2022, 87, 6961-7055

2021
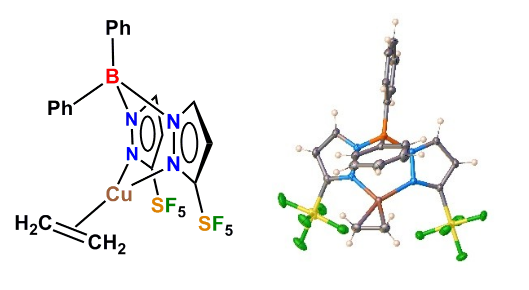
143) When SF5 outplays CF3: Effects of pentafluorosulfanyl decorated scorpionates on copper
A. Noonikara Poyil, A. Muñoz-castro, A. Boretskyi, P. K. Mykhailiuk,* R. Dias*
Chem. Sci. 2021, 12, 14618-14623
142) Oxa-spirocycles: synthesis, properties and applications
K. Fominova, T. Diachuk, D. Granat, T. Savchuk, V. Vilchynskyi, O. Svitlychnyi, V. Meliantsev, I. Kovalchuk, E. Litskan, V. V. Levterov, V. R. Badlo, R. I. Vaskevych, A. I. Vaskevych, A. V. Bolbut, V. V. Semeno, R. Iminov, K. Shvydenko, A. S. Kuznetsova, Y. V. Dmytriv, D. Vysochyn, V. Ripenko, A. A. Tolmachev, O. Pavlova, H. Kuznietsova, I. Pishel, P. Borysko, P. K. Mykhailiuk*
Chem. Sci. 2021, 12, 11294-11305

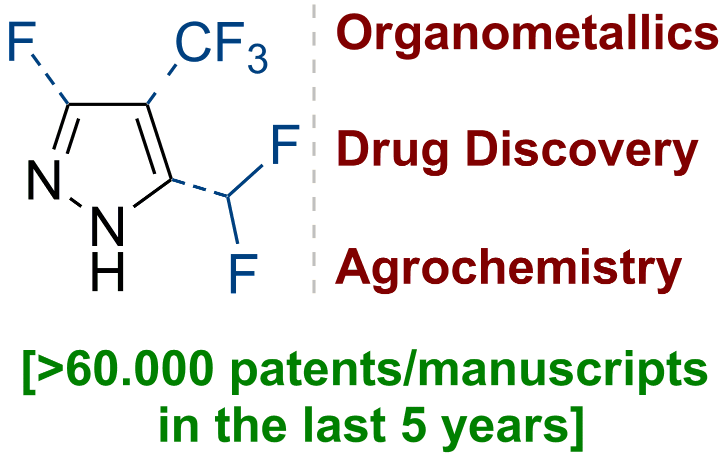
141) Fluorinated Pyrazoles: From Synthesis to Applications
P. K. Mykhailiuk*
Chem. Rev. 2021, 121, 1670-1715
140) Large-Scale Synthesis and Modifications of Bicyclo[1.1.1]pentane-1,3-dicarboxylic Acid (BCP)
V. Ripenko, D. Vysochyn, I. Klymov, S. Zhersh, P. K. Mykhailiuk*
J. Org. Chem. 2021, 86, 14061-14068
![Large-Scale Synthesis and Modifications of Bicyclo[1.1.1]pentane-1,3-dicarboxylic Acid (BCP)](https://mykhailiukchem.org/wp-content/uploads/2021/11/140.png)
139) Bicyclic Pyrrolidines for Medicinal Chemistry via [3 + 2]-Cycloaddition
V. I. Savych, V. L. Mykhalchuk, P. V. Melnychuk, A. O. Isakov, T. Savchuk, V. M. Timoshenko, S. A. Siry, S. O. Pavlenko, D. V. Kovalenko, O. V. Hryshchuk, V. A. Reznik, B. A. Chalyk, V. S. Yarmolchuk, E. B. Rusanov, P. K. Mykhailiuk*
J. Org. Chem. 2021, 86, 13289-13309
![Bicyclic Pyrrolidines for Medicinal Chemistry via [3 + 2]-Cycloaddition](https://mykhailiukchem.org/wp-content/uploads/2021/11/139.png)
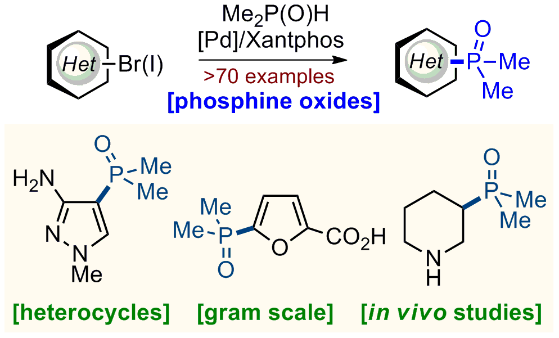
138) Phosphine Oxides (−POMe2) for Medicinal Chemistry: Synthesis, Properties, and Applications
M. V. Stambirskyi, T. Kostiuk, S. I. Sirobaba, A. Rudnichenko, D. L. Titikaiev, Y. V. Dmytriv, H. Kuznietsova, I. Pishel, P. Borysko, P. K. Mykhailiuk*
J. Org. Chem. 2021, 86, 12783-12801
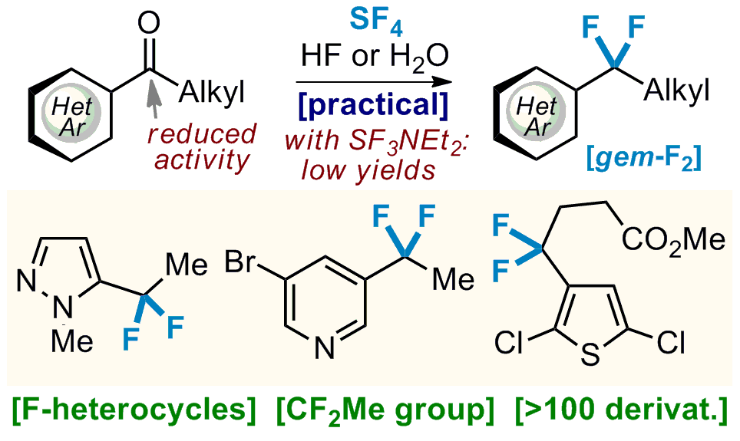
137) Scalable Approach to Fluorinated Heterocycles with Sulfur Tetrafluoride (SF4)
S. Trofymchuk, M. Bugera, A. A. Klipkov, V. Ahunovych, B. Razhyk, S. Semenov, A. Boretskyi, K. Tarasenko, P. K. Mykhailiuk*
J. Org. Chem. 2021, 86, 12181-12198
136) Bicyclic Piperidines via [2 + 2] Photocycloaddition
V. Shcherbakova, D. Dibchak, M.Snisarenko, Y. Skalenko, A. V. Denisenko, A. S. Kuznetsova, P. K. Mykhailiuk*
J. Org. Chem. 2021, 86, 2200-2209
![Bicyclic Piperidines via [2 + 2] Photocycloaddition](https://mykhailiukchem.org/wp-content/uploads/2021/11/136.png)










Comments are closed.