
1. Chemistry of diazo compounds
In 2014-17, we developed three novel chemical reagents: difluoromethyl diazomethane (CHF2CHN2), pentafluoroethyl diazomethane (C2F5CHN2), and heptafluoroisopropyl diazomethane (i-C3F7CHN2). We also work with trifluoromethyl diazomethane (CF3CHN2) and cyano diazomethane (CNCHN2). We generate them in situ, and study their reactivity as nucleophiles, electrophiles, in cyclopropanation, carbene insertions, and [3+2]-cycloaddition reactions.

2. 3D-Shaped scaffolds for drug discovery
The cores of pyrrolidine and piperidine comprise to the structure of more than 100 FDA-approved drugs. In the loop of the recent concepts scaffold hopping, escape the flatland and conformational restriction, medicinal chemists now tend to use now Fsp3-rich bicyclic building blocks. We therefore develop practical synthetic approaches to novel bicyclic analogues of pyrrolidine/piperidine: spirocycles, fused and bridged amines. We incorporate these structures into drugs, and study the biological properties. For example, the spirocyclic analogue of the local anesthetic Bupivacaine was more active than the original drug.

3. Photochemistry
To synthesize bicyclic building blocks for drug discovery, we often use photochemistry. On a gram scale we perform the photochemical syntheses in batch, while on a kg scale – in flow.

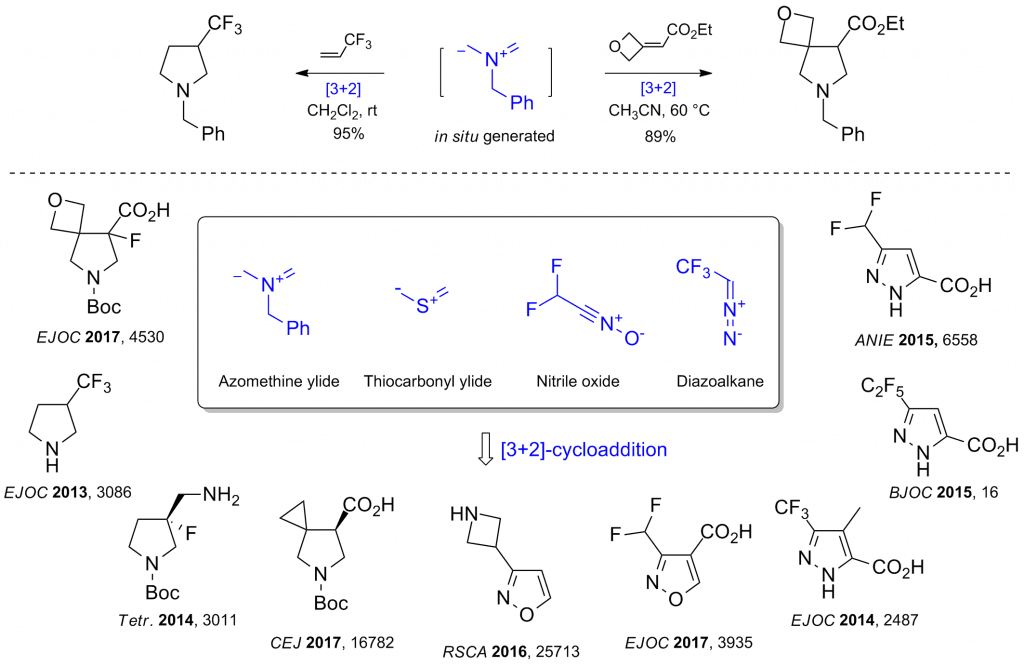
4. [3+2]-Cycloadditions
We also exploit [3+2]-cycloadditions of azomethine ylides, thicarbonyl ylides, nitriloxides and diazo alkanes to synthesize the pharmacologically relevant five-membered heterocycles: pyrrolidines, tetrahydrothiophenes, isoxazoles, isoxazolines, pyrazoles and pyrazolines.
5. Fluorinated amines
Incorporation of fluorine atoms into organic compounds can increase their metabolic stability, and decrease the basicity of the neighboring nitrogen atom. Therefore, we have synthesized a library of novel fluorinated amines for drug discovery: CF3-azetidine, CF3-pyrrolidine and CF3-morpholine. The key step in many syntheses was the transformation of the carboxylic group into the trifluoromethyl one by SF4.

6. Synthesis of analogues of L-Proline
We synthesize the novel fluorinated and conformationally restricted analogues of L-Proline. We also study their conformational behavior. For example, derivatives of 2,4-methanoproline stabilize N-trans amide bond: Ktrans/cis > 100. Conformationally restricted prolines are interesting as building blocks for drug discovery, and the fluorinated ones also as 19F-labels to study the peptides.

7. C-H activation of cyclic amino acids
New 3D-shaped F(sp3)-rich molecules can be easily accessed by the CH-activation/hydrolysis of the appropriately protected cyclic α-amino acids.

Comments are closed.