
2025
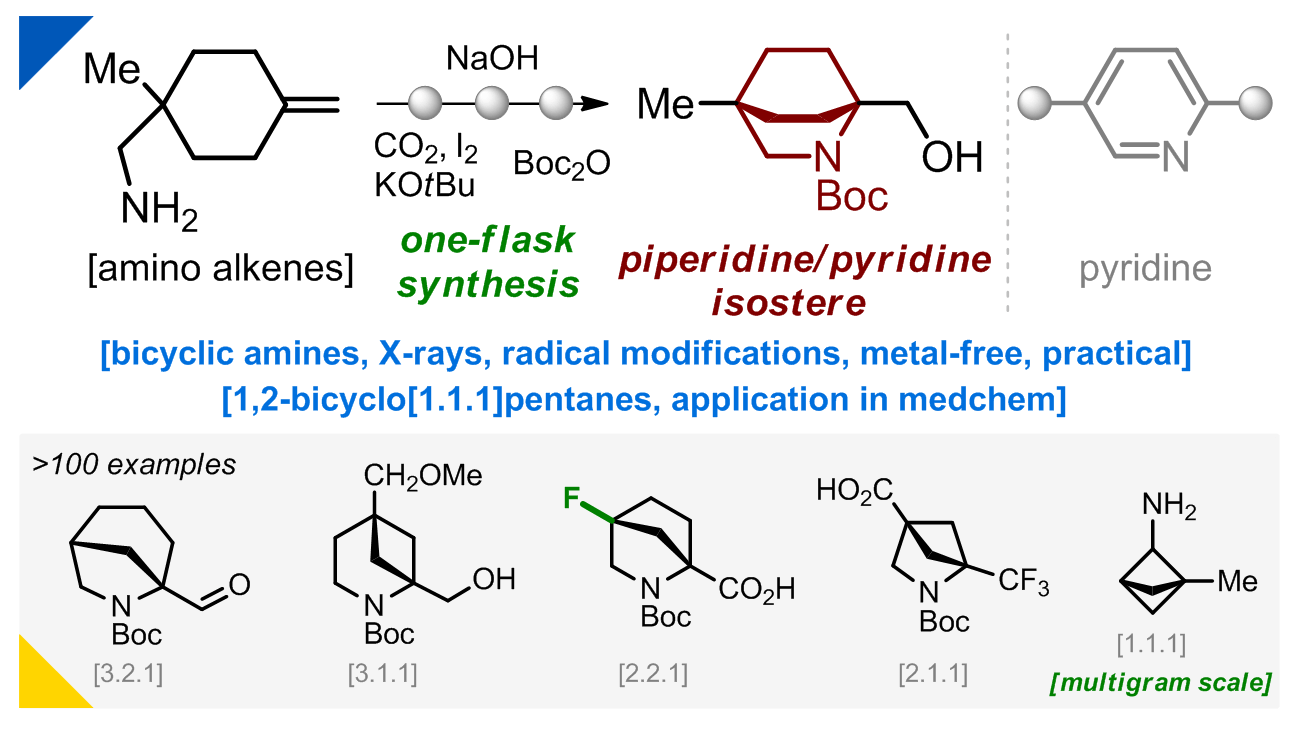
186) Bicyclic Isosteres of Pyridine/Piperidine: From Synthesis to Applications
O. Stashkevych, V. Kokhalskyi, Y. Mynak, V. Levterov, O. Shablykin, I. Pishel, P. Mykhailiuk*
Angew. Chem. Int. Ed. 2025, 64, e202517814
185) 3-Oxabicyclo[3.1.1]heptane as an Isostere of meta-Benzene
D. Dibchak, P. K. Mykhailiuk*
Angew. Chem. Int. Ed. 2025, 64, e202505519
![3-Oxabicyclo[3.1.1]heptane as an Isostere of meta-Benzene](https://mykhailiukchem.org/wp-content/uploads/2025/11/185.png)
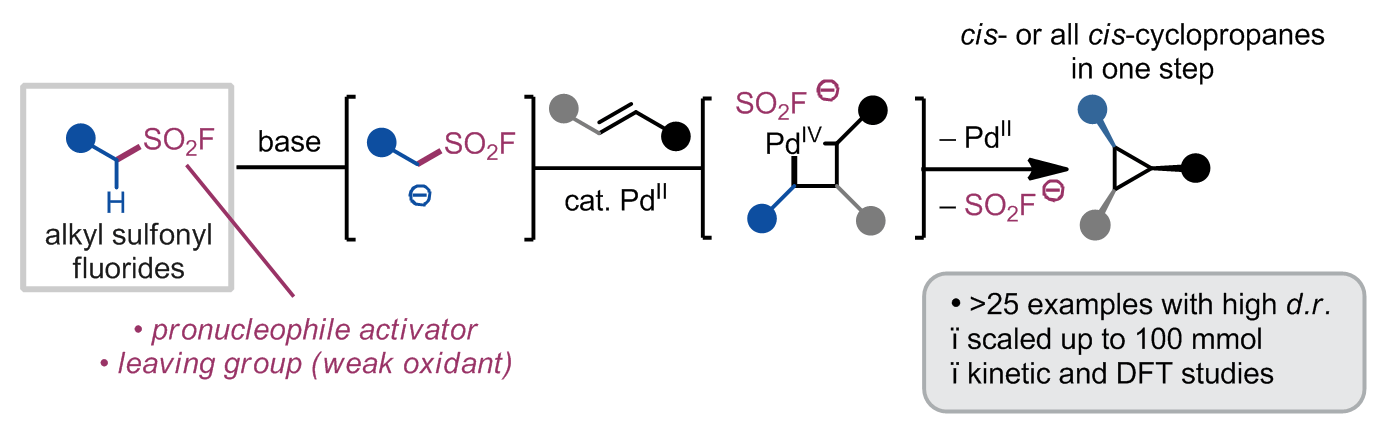
184) Alkyl sulfonyl fluorides as ambiphiles in the stereoselective palladium(II)-catalysed cyclopropanation of unactivated alkenes
Y. Cao, W. Rodphon, T. M. Alturaifi, A. V. R. D. Lisboa, Z. Ren, J. J. C. Struijs, H.-Q. Ni, T. Savchuk, R. P. Loach, S. Yang, I. J. McAlpine, D. G. Blackmond, P. K. Mykhailiuk,* P. Liu,* K. B. Sharpless,* K. M. Engle*
Nature Synth. 2025, accepted
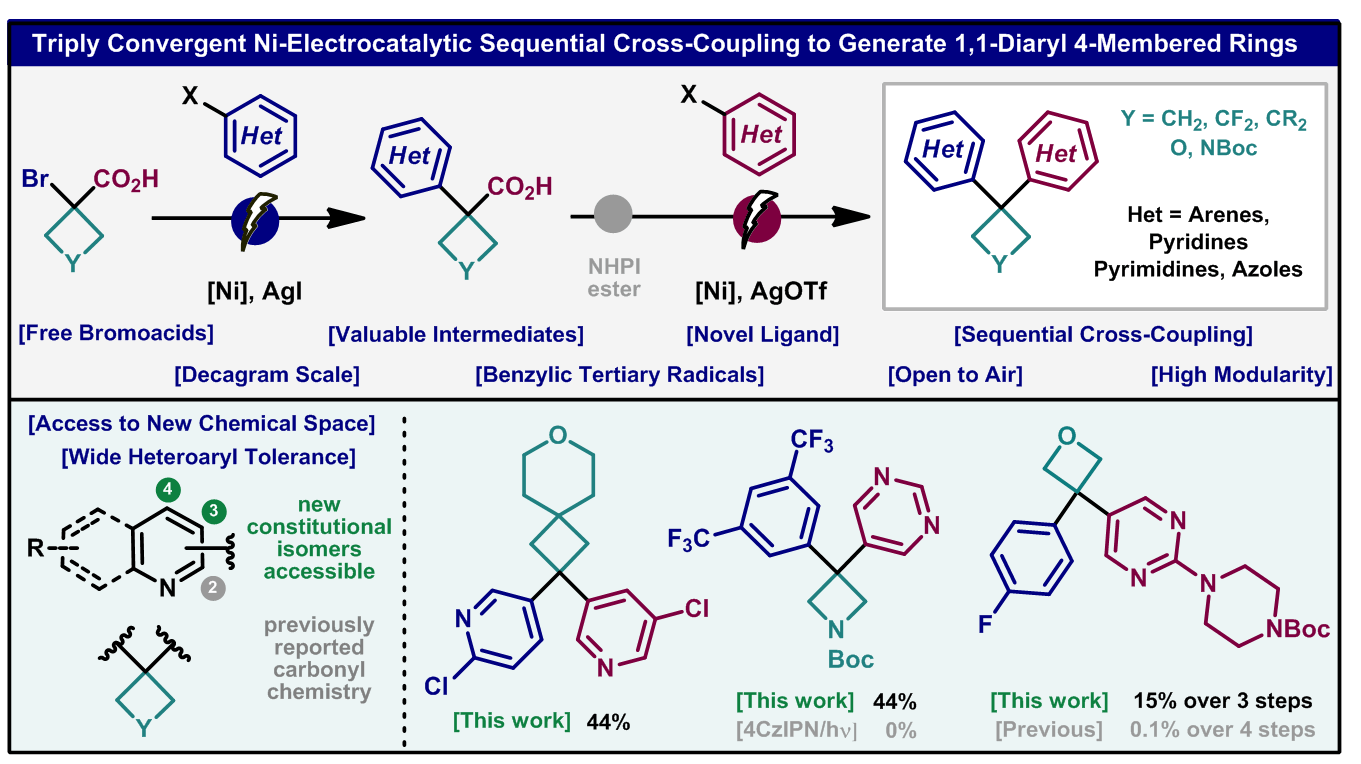
183) Triply convergent Ni-electrocatalytic assembly of 1,1-diaryl cyclobutanes, azetidines and oxetanes
L. Massaro, P. Neigenfind, A. Feng, G. Kuehn, F. C. Attard, A. DeSanti, M. R. Collins, M. Bravo, R. K. Twumasi, D. Chen, P. N. Bolduc, M. Nicastri, M. A. Emmanuel, M. S. Oderinde, M. D. Palkowitz, X. Zheng, A. C. Hunter, K. C. Harper, C. C. Tyrol, P. K. Mykhailiuk, Y. Kawamata, P. S. Baran*
Nature Chem. 2025, in press
182) Harnessing O‑Vinylhydroxylamines for Ring-Annulation: A Scalable Approach to Azaindolines and Azaindoles
Z. Grimm, C. Randolph, O. Buravov, P. Mykhailiuk,* L. Kürti*
J. Am. Chem. Soc. 2025, 147, 27148–27154

181) Dibromocarbene Addition to Bicyclo[1.1.0]butanes: A Facile Route to Substituted Bicyclo[1.1.1]pentanes
F. C. Attard, A. Slobodianyk, R. Bychek, Y. Panasiuk, P. Neigenfind, L. Massaro, M. G. Gardiner, V. V. Levterov, P. S. Baran,* P. K. Mykhailiuk,* L. R. Malins*
Proc. Natl. Acad. Sci. USA 2025, 122, e2524130122
![Dibromocarbene Addition to Bicyclo[1.1.0]butanes: A Facile Route to Substituted Bicyclo[1.1.1]pentanes](https://mykhailiukchem.org/wp-content/uploads/2025/11/181.png)
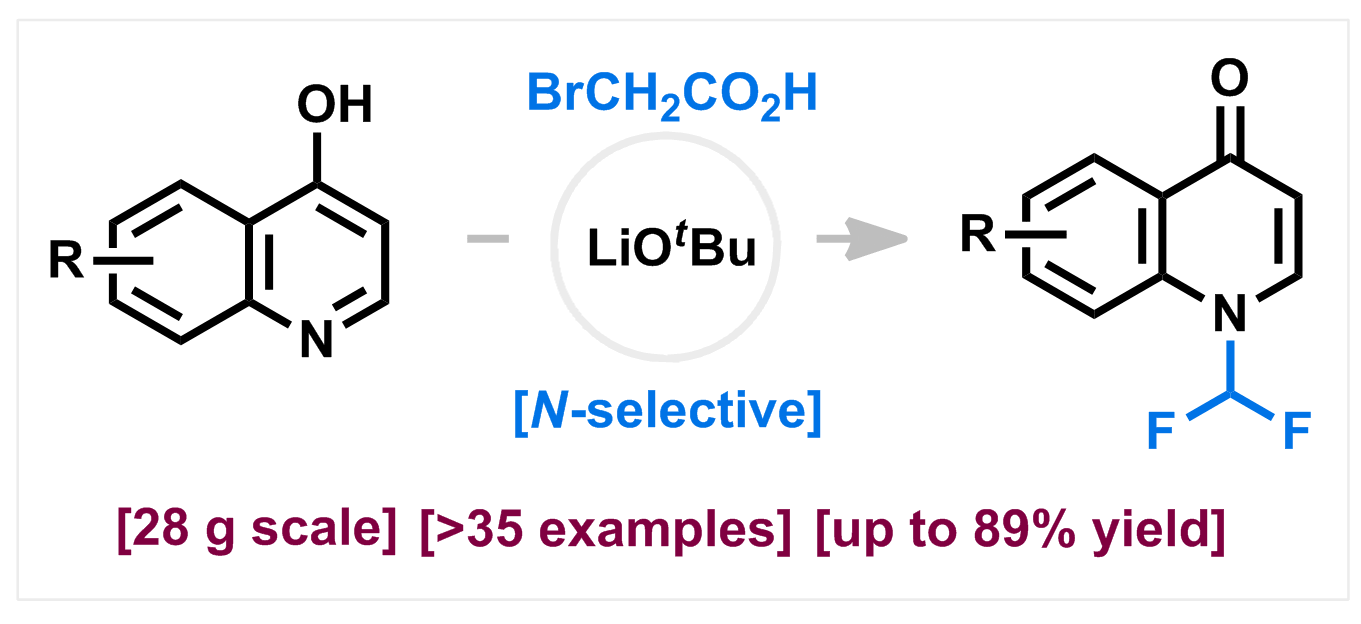
180) N‑Selective Difluoromethylation of 4‑Hydroxyquinolines
S. Kumawat, G. N. Nair, V. N. Kalevaru, S. Wohlrab, T. Tarasiuk, T. Yegorova, P. Mykhailiuk,* K. Natte*
Org. Lett. 2025, 27, 11466–11473
179) An Approach to Aliphatic Sulfonyl Fluorides
P. Dychkova, O. P. Datsenko, I. Sadkova, P. K. Mykhailiuk*
Org. Lett. 2025, 27, 10489–10493
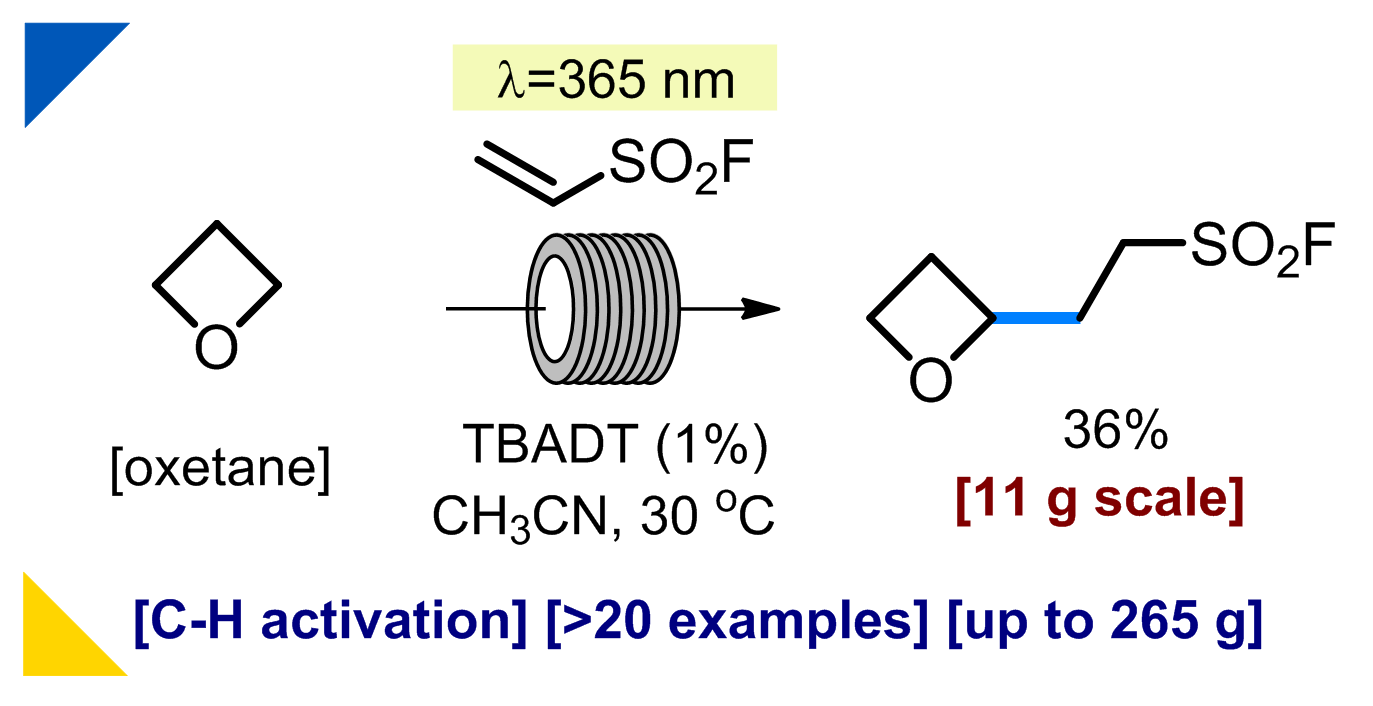
178) Approach to Heterospirocycles for Medicinal Chemistry
C. Rodriguez-Arias, R. Miguelez, Y. Holota, P. K. Mykhailiuk,* P. Barrio*
Org. Lett. 2025, 27, 10342–10347

177) P(O)Me2–Alkenes: From Synthesis to Applications
P. V. Melnychuk, O. V. Kulish, S. I. Pavlish, V. Kubyshkin, P. K. Mykhailiuk*
Org. Lett. 2025, 27, 6174–6178

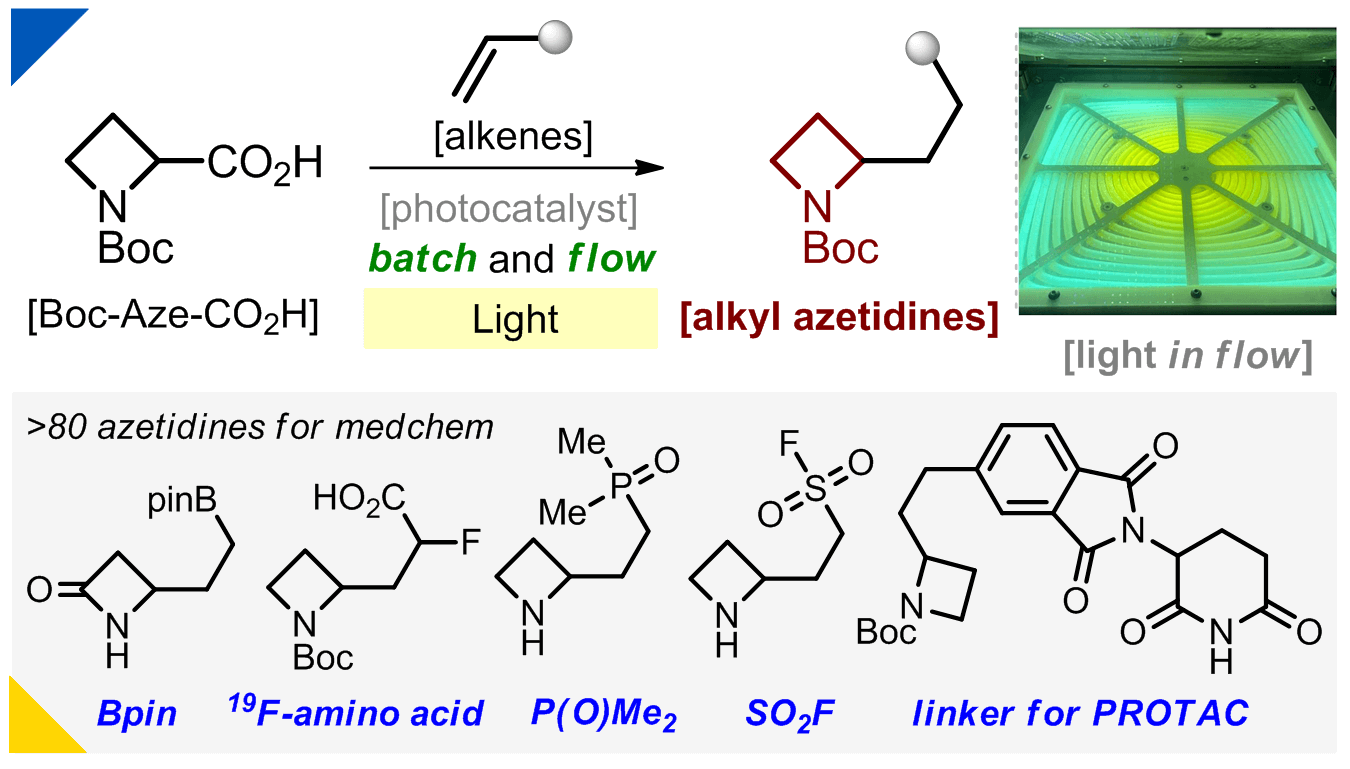
176) Alkyl Azetidines Via Batch and Flow Photochemistry
O. P. Datsenko, A. Baziievskyi, I. Sadkova, B. Campos, J. T. Brewster II*, J. Kowalski, R. J. Hinklin*, P. K. Mykhailiuk*
Org. Lett. 2025, 27, 5318–5323
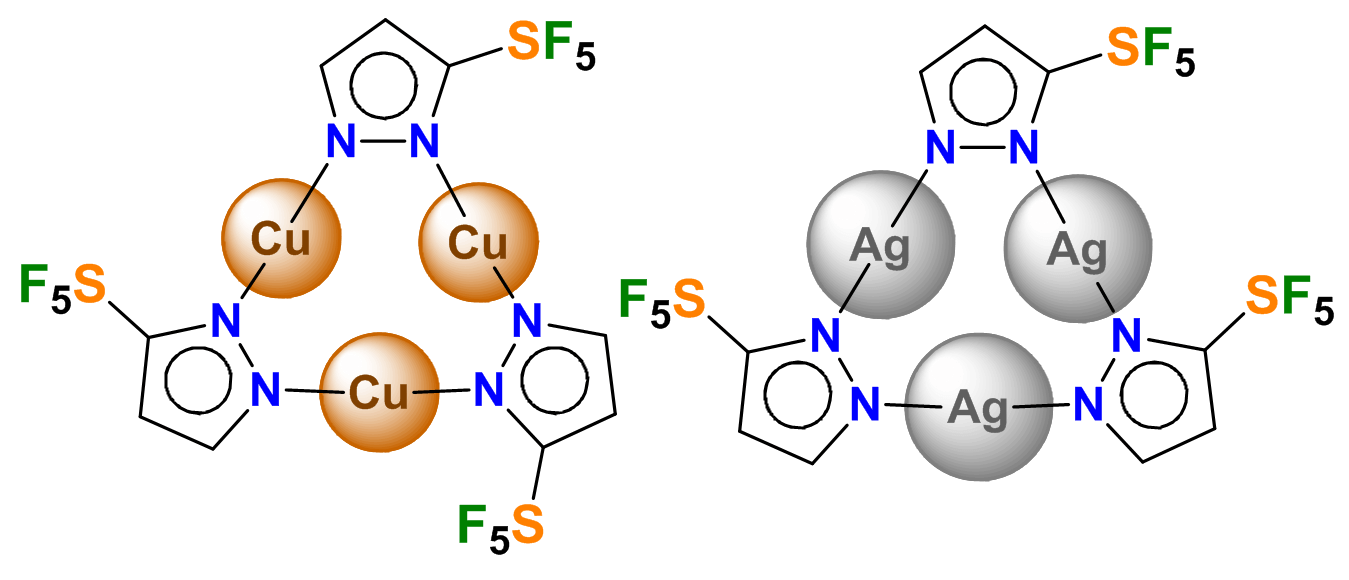
175) Trinuclear Copper(I) and Silver(I) Complexes of a Pentafluorosulfanyl-Decorated Pyrazolate
M. Vanga, B. T. Diroll,* A. Muñoz-Castro,* A. Boretskyi, P. Mykhailiuk,* H. V. Rasika Dias*
Inorg. Chem. 2025, in press
174) A Reagent to Access Methyl Sulfones
Y. Poplavskyi, V. Ripenko, S. Bova, A. Biitseva, Y. V. Dmitriv, A. A. Tolmachev, I. V. Sadkova, I. Pishel, O. Grygorov, V. Q. H. Phan, H. V. R. Dias,* P. K. Mykhailiuk*
Nature Commun. 2025, 16, 1132

173) Saturated F2-Rings from Alkenes
Y. Li, X.-B. Liu, V. Sham, I. Logvinenko, J.-H. Xue, J.-Y. Wu, J.-L. Fu, S. Lin, Y. Liu, Q. Li,* P. K. Mykhailiuk,* H. Wang*
Angew. Chem. Int. Ed. 2025, 64, e202422899

172) “Angular” spirocyclic azetidines: synthesis, characterization, and evaluation in drug discovery
A. A. Kirichok, H. Tkachuk, K. Levchenko, D. Granat, T. Yegorova, D. Lesyk, A. Anisiforova, Y. Holota, V. Zomchak, I. Bodenchuk, V. Kosach, P. Borysko, R. A. Korzh, G. Al-Maali, V. Kubyshkin, H. S. Rzepa, P. K. Mykhailiuk*
Angew. Chem. Int. Ed. 2025, 64, e202418850

2024
171) Light-enabled scalable synthesis of bicyclo[1.1.1]pentane halides and their functionalizations
V. Ripenko, V. Sham, V. Levchenko, S. Holovchuk, D. Vysochyn, I. Klymov, D. Kyslyi, S. Veselovych, S. Zhersh, Y. Dmytriv, A. Tolmachev, I. Sadkova, I. Pishel, K. Horbatok, V. Kosach, Y. Nikandrova, P. K. Mykhailiuk*
Nature Synth. 2024, 3, 1538–1549
![Light-enabled scalable synthesis of bicyclo[1.1.1]pentane halides and their functionalizations](https://mykhailiukchem.org/wp-content/uploads/2024/11/171.png)
170) CF3-Cyclobutanes: Synthesis, Properties, and Evaluation as a Unique tert-Butyl Group Analogue
V. Ahunovych, A. A. Klipkov, M. Bugera, K. Tarasenko, S. Trofymchuk, B. Razhyk, A. Boretskyi, O. Stanko, Y. Panasiuk, O. Shablykin, G. Al-Maali, D. Lesyk, O. Klymenko-Ulianov, K. Horbatok, I. Bodenchuk, V. Kosach, P. Borysko, V. Kubyshkin, P. K. Mykhailiuk*
JACS Au 2024, 4, 4507–4517

169) Proline Analogues in Drug Design: Current Trends and Future Prospects
V. Kubyshkin,* P. K. Mykhailiuk*
J. Med. Chem. 2024, 67, 20022–20055

168) Strain-Release-Driven Modular Synthesis of Oxetane-Based Amide Bioisosteres: Concise, Robust and Scalable Approach
P. Spránitz, P. Sőregi, K. Hegedüs, B. Igriczi, G. Szakács, K. Jemnitz, P. Szabó, Y. Galushchak, P. K. Mykhailiuk, T. Soós*
Angew. Chem. Int. Ed. 2024, 63, e202410554

167) Functionalization of Alkenes with Difluoromethyl Nitrile Oxide to Access the Difluoromethylated Derivatives
B. A. Chalyk, O. Zginnyk, A. V. Khutorianskyi, P. K. Mykhailiuk*
Org. Lett. 2024, 26, 2888–2892

165) Bicyclo[m.n.k]alkane Building Blocks as Promising Benzene and Cycloalkane Isosteres: Multigram Synthesis, Physicochemical and Structural Characterization
V. V. Semeno, V. O. Vasylchenko, I. M. Fesun, L. Y. Ruzhylo, M. O. Kipriianov, K. P. Melnykov, A. Skreminskyi, R. Iminov, P. Mykhailiuk, B. V. Vashchenko, O. O. Grygorenko*
Chem. Eur. J. 2024, 24, e202303859
![Bicyclo[m.n.k]alkane Building Blocks as Promising Benzene and Cycloalkane Isosteres: Multigram Synthesis, Physicochemical and Structural Characterization](https://mykhailiukchem.org/wp-content/uploads/2024/11/165.png)
164) 2-Oxabicyclo[2.1.1]hexanes: synthesis, properties and validation as bioisosteres of ortho- and meta-Benzenes
V. V. Levterov, Y. Panasiuk, O. Shablykin, O. Stashkevych, K. Sahun, A. Rassokhin, I. Sadkova, D. Lesyk, A. Anisiforova, Y. Holota, P. Borysko, I. Bodenchuk, N. M. Voloshchuk, P. K. Mykhailiuk*
Angew. Chem. Int. Ed. 2024, 63, e202319831
![2-Oxabicyclo[2.1.1]hexanes: synthesis, properties and validation as bioisosteres of ortho- and meta-Benzenes](https://mykhailiukchem.org/wp-content/uploads/2024/02/164.png)
163) Photosensitization Enables Pauson-Khand-type Reactions with Nitrenes
F. Li, W. F. Zhu, C. Empel, O. Datsenko, A. Kumar, Y. Xu, J. H. M. Ehrler, I. Atodiresei, S. Knapp, P. K. Mykhailiuk, E. Proschak, R. M. Koenigs*
Science 2024, 383, 498

162) Spiro[3.3]heptane as a Saturated Benzene Bioisostere
K. Prysiazhniuk, O. P. Datsenko, O. Polishchuk, S. Shulha, O. Shablykin, Y. Nikandrova, K. Horbatok, I. Bodenchuk, P. Borysko, D. Shepilov, I. Pishel, V. Kubyshkin, P. K. Mykhailiuk*
Angew. Chem. Int. Ed. 2024, 63, e202316557
![Spiro[3.3]heptane as a Saturated Benzene Bioisostere](https://mykhailiukchem.org/wp-content/uploads/2024/02/162.png)
161) Nickel-Electrocatalytic Decarboxylative Arylation to Access Quaternary Centers
G. Laudadio, P. Neigenfind, Á. Péter, C. Z. Rubel, M. A. Emmanuel, M. S. Oderinde, T. El-Hayek Ewing, M. D. Palkowitz, J. L. Sloane, K. W. Gillman, D. Ridge, M. D. Mandler, P. N. Bolduc, M. C. Nicastri, B. Zhang, S. Clementson, N. N. Petersen, P. Martín-Gago, P. Mykhailiuk, K. M. Engle,* P. S. Baran*
Angew. Chem. Int. Ed. 2024, 63, e202314617

160) Borylated cyclobutanes via thermal [2 + 2]-cycloaddition
K. Prysiazhniuk, O. Polishchuk, S. Shulha, K. Gudzikevych, O. P. Datsenko, V. Kubyshkin, P. K. Mykhailiuk*
Chem. Sci. 2024, 15, 3249-3254
![Borylated cyclobutanes via thermal [2 + 2]-cycloaddition](https://mykhailiukchem.org/wp-content/uploads/2024/02/160.png)
159) In situ studies of reversible solid–gas reactions of ethylene responsive silver pyrazolates
H. V. R. Dias, D. Parasar, A. A. Yakovenko, P. W. Stephens, Á. Muñoz-Castro, M. Vanga, P. Mykhailiuk, E. Slobodyanyuk
Chem. Sci. 2024, 15, 2019-2025



Comments are closed.